Stars are the glowing furnaces of the universe, converting simple particles into complex elements through nuclear fusion. But to truly understand how stars work, we first need to explore the building blocks of matter itself—atoms and their subatomic components. In this lesson, we’ll journey into the heart of the atom, uncover the forces that hold it together, and examine the role that particles play in the lives of stars.
Video
Watch this video to gain a visual understanding of atomic structures.
Introduction to Atomic Structure
Atoms are the fundamental units of matter, making up everything in the universe, from the air we breathe to the stars we observe in the night sky. But what exactly are atoms composed of?
Protons, Neutrons, and Electrons
Atoms consist of three primary subatomic particles: protons, neutrons, and electrons. These particles are responsible for the identity and behavior of atoms, including their role in stellar processes like nuclear fusion.
- Protons: Positively charged particles found in the nucleus of an atom. The number of protons determines the element’s identity.
- Neutrons: Neutral particles also located in the nucleus. They provide stability to the nucleus by balancing the repulsive forces between protons.
- Electrons: Negatively charged particles that orbit the nucleus. Electrons are responsible for chemical bonds and the behavior of atoms in different states of matter.
Table of Subatomic Particles
| Particle | Mass (kg) | Charge | Location |
|---|---|---|---|
| Proton | $1.6726 \times 10^{-27}$ | +1 (positive) | Nucleus |
| Neutron | $1.6749 \times 10^{-27}$ | 0 (neutral) | Nucleus |
| Electron | $9.1094 \times 10^{-31}$ | -1 (negative) | Orbiting nucleus |
Atomic Number and Mass Number
- Atomic Number: The number of protons in the nucleus of an atom, which defines the element.
- Mass Number: The total number of protons and neutrons in an atom’s nucleus.
For example, carbon has an atomic number of 6 and a typical mass number of 12, meaning it has 6 protons and 6 neutrons.

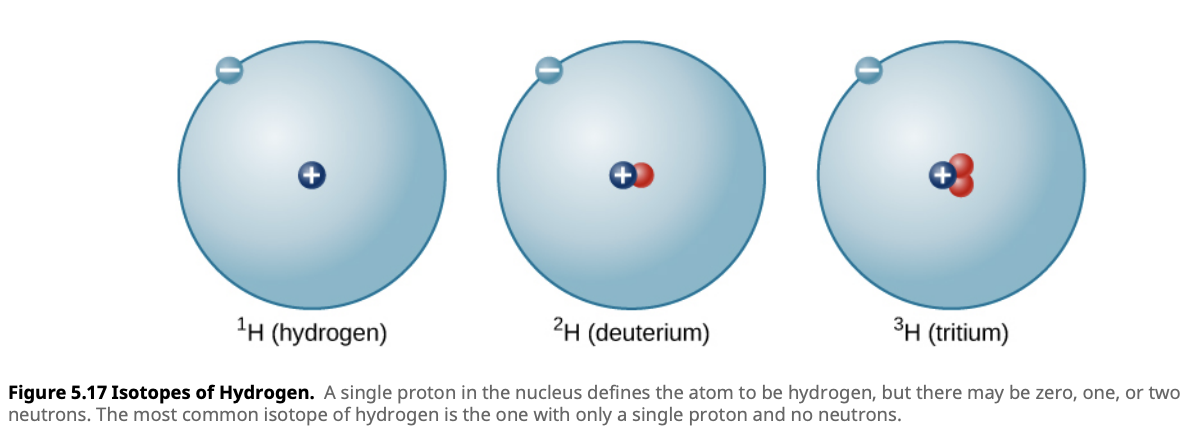
Isotopes
Isotopes are variations of an element that have the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive and can decay over time. In stars, isotopes play a critical role in nuclear fusion processes, where atoms combine to form heavier elements, releasing vast amounts of energy.
Did You Know? The Sun primarily fuses hydrogen isotopes (protons) to form helium, providing the energy that powers our solar system.
Neutrinos and Photons
Now, let’s explore two additional key particles—neutrinos and photons—that play critical roles in both atomic and stellar processes.
Neutrinos
Neutrinos are incredibly light, neutral particles that interact very weakly with matter. Produced in vast quantities during nuclear reactions in stars, neutrinos can pass through entire planets without interacting with any matter. These elusive particles are critical in nuclear fusion processes in stars and play a key role in supernovae explosions, carrying away much of the energy released.
Fun Fact: Every second, trillions of neutrinos from the Sun pass through your body unnoticed!

Photons
Photons are the basic units of light and all other forms of electromagnetic radiation. They are massless particles that travel at the speed of light and carry energy. In stars, photons are produced during nuclear fusion reactions and are responsible for the light and heat that stars emit.
The Nature of Light: Wave-Particle Duality
Light exhibits both wave-like and particle-like properties. As a wave, light can be described by its wavelength and frequency. As a particle, light is made up of photons. This wave-particle duality is a fundamental concept in quantum physics and helps explain the behavior of light in different situations, such as when it passes through a prism or interacts with matter.
Fun Fact: Photons from the Sun take hundreds of thousands of years to escape from the Sun’s core to its surface, but only 8 minutes to travel the 150 million kilometers to Earth.
Table of Electromagnetic Radiation
| Type of Radiation | Wavelength Range (nm) | Radiated by Objects at This Temperature | Typical Sources |
|---|---|---|---|
| Gamma rays | Less than 0.01 | More than $10^8$ K | Produced in nuclear reactions; require very high-energy processes |
| X-rays | 0.01–20 | $10^6$–$10^8$ K | Gas in clusters of galaxies, supernova remnants, solar corona |
| Ultraviolet | 20–400 | $10^4$–$10^6$ K | Supernova remnants, very hot stars |
| Visible | 400–700 | $10^3$–$10^4$ K | Stars |
| Infrared | $10^3$–$10^6$ | 10–$10^3$ K | Cool clouds of dust and gas, planets, moons |
| Microwave | $10^6$–$10^9$ | Less than 10 K | Active galaxies, pulsars, cosmic background radiation |
| Radio | More than $10^9$ | Less than 10 K | Supernova remnants, pulsars, cold gas |
Forces within Atoms
Atoms are held together through the interaction of the four fundamental forces. Let’s explore these forces and their roles in both atomic structure and stellar processes.
The Electromagnetic Force
The electromagnetic force is responsible for the attraction between negatively charged electrons and positively charged protons, keeping electrons in orbit around the nucleus. This force also governs the behavior of charged particles in stellar environments, such as plasma. Additionally, the electromagnetic force is responsible for the repulsion of like charges, such as the repulsive force between two positively charged protons within an atom’s nucleus.
The Strong Nuclear Force
The strong nuclear force is what holds protons and neutrons together in the nucleus, despite the repulsive electromagnetic force between the positively charged protons. This force is incredibly powerful but acts only at very short distances within the nucleus.
The Weak Nuclear Force
The weak nuclear force is involved in processes like beta decay, where a neutron can transform into a proton, emitting a neutrino and an electron. This force plays a significant role in the nuclear reactions that occur in stars, particularly during the fusion of lighter elements into heavier ones.
Gravity
While gravity is the weakest of the four fundamental forces on the atomic scale, it plays a crucial role in the formation and life cycles of stars. Gravity is the force that causes molecular clouds to collapse, initiating the star formation process, and it governs the structure of stars throughout their lifetimes.
Radioactive Decay
Radioactive decay is a natural process in which unstable atomic nuclei lose energy by emitting radiation. This process transforms atoms into different elements or isotopes over time. In stellar environments, radioactive decay plays a crucial role in the production of energy and the synthesis of new elements, contributing to the life cycles of stars and the evolution of the universe.
Types of Radioactive Decay
- Alpha Decay: In this type of decay, the nucleus emits an alpha particle (two protons and two neutrons), reducing the mass number of the atom.
- Beta Decay: During beta decay, a neutron is converted into a proton, and an electron (beta particle) and a neutrino are emitted.
- Gamma Decay: Gamma decay occurs when the nucleus releases energy in the form of gamma rays, without changing the number of protons or neutrons.
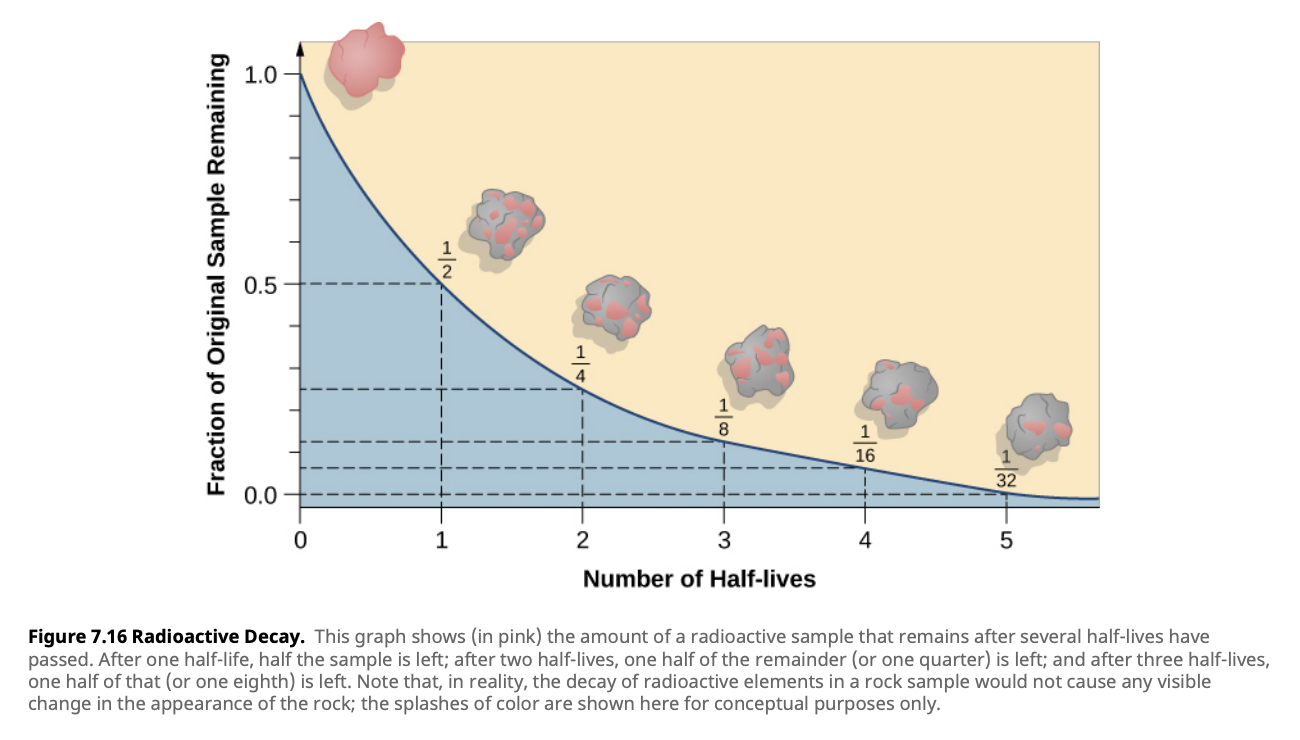
Half-Life and Its Significance
The half-life of a radioactive isotope is the time it takes for half of the atoms in a sample to decay. Half-lives can range from fractions of a second to billions of years. In stars, radioactive decay processes contribute to the production of energy and the creation of new elements.
Example: Uranium-238, with a half-life of 4.5 billion years, plays a key role in the heat generation inside planets like Earth.
Here’s an enhanced version of the “Particles in Stellar Environments” section:
Particles in Stellar Environments
The universe’s most powerful laboratories are stars, where temperatures and pressures soar to unimaginable extremes. These harsh environments cause particles to behave in ways that defy our everyday experiences on Earth.
High-Energy Environments in Stars
At the heart of a star, the temperature can exceed millions of degrees Celsius—hot enough to strip atoms of their electrons. This creates a plasma, an incredibly energetic state of matter composed of freely moving ions and electrons. Plasma is often described as a “soup” of charged particles, and in this environment, particles collide at incredible speeds, facilitating nuclear fusion reactions that power the star.
As these particles interact, they generate intense magnetic fields and release vast amounts of energy in the form of light and heat. The movement of charged particles in this plasma also generates phenomena such as solar flares, which can burst out from the star’s surface with explosive force, affecting entire planetary systems.
Fun Fact: Plasma, the most common state of matter in the universe, makes up 99% of all visible matter, including stars, lightning, and even neon signs.
Stellar Winds and Supernovae
Stars are not just passive energy emitters—they are also sources of powerful particle flows that shape the universe around them.
-
Stellar Winds: Many stars, particularly massive ones, emit continuous streams of charged particles known as stellar winds. These winds are composed mainly of protons and electrons and can extend far beyond the star, influencing the formation of planets, asteroids, and even entire star systems. Stellar winds can erode the outer layers of a dying star, contributing to the creation of stunning nebulae—vast clouds of gas and dust that may eventually collapse to form new stars.
Stellar winds are also responsible for the breathtaking beauty of planetary nebulae and the majestic structures seen in star-forming regions like the Eagle Nebula’s “Pillars of Creation.”

-
Supernovae: The death throes of a massive star culminate in a supernova—a colossal explosion that outshines entire galaxies. During a supernova, a star ejects enormous quantities of material into space at speeds approaching the speed of light. These high-energy particles, accelerated by the explosion, become cosmic rays that travel through the galaxy, bombarding planets and influencing their atmospheres.
Supernovae are also nature’s forge for the heaviest elements, including iron, gold, and uranium. The very atoms that make up your body, the Earth, and everything around you were once created in the fiery death of a star. Without supernovae, the universe would lack the heavy elements necessary for life.
Check Your Understanding
-
Atomic Structure: Explain how the atomic number and mass number define the identity and characteristics of an element. How do these properties influence the formation of isotopes in stellar environments?
-
Subatomic Particles: Compare and contrast the roles of protons, neutrons, and electrons in atomic structure. How do their respective masses and charges contribute to the stability and reactivity of an atom?
-
Forces within Atoms: Describe the strong nuclear force and explain why it is crucial in holding the nucleus together, despite the repulsive electromagnetic force between protons. How does this force compare to gravity on an atomic scale?
-
Neutrinos: Why are neutrinos so important in the study of stars, and why do you think they are so difficult to detect?
-
Photons: What are photons, and how do they contribute to the energy emitted by stars?
-
Electromagnetic Radiation: Examine the different types of electromagnetic radiation (gamma rays, X-rays, ultraviolet, visible light, etc.) and their relationship to the temperature and behavior of astronomical objects. How do these radiation types help astronomers study the universe?
Resources
- Astronomy (2016). Andrew Fraknoi, David Morrison, and Sidney C. Wolff.
- Foundations of Astrophysics (2010). Barbara Ryden and Bradley M. Peterson.